In pharmaceutical and nutraceutical manufacturing, capsule filling must deliver repeatable dosing and reliable closure quality at production throughput. Fully automatic hard capsule filling machines achieve this by running a fixed station sequence—orientation, cap/body separation, metering, closing, and discharge—synchronized by turret timing and control logic.
This guide explains the capsule filling machine working principle at station level, so you can see what each module controls, what conditions destabilize the cycle, and which checks (weight trend, closure consistency, reject patterns) show the run is in control.

A fully automatic cycle repeats the same operations in the same order:
1. Rectify (orient) capsules
2. Separate cap and body
3. Meter and transfer the dose
4. Close and lock
5. Discharge and reject
“Stable performance” should mean the station cycle stays consistent over time—not only that the machine reaches a headline speed.
Manual systems suit trials and small batches; control is largely operator-driven. Semi-automatic machines automate parts of the flow but remain interrupted and less comparable to continuous station control. This article assumes fully automatic capsule filling machines: turret-based, continuous cycles, integrated sensing, and reject logic.
● Rectification: aligning capsules for correct entry.
● Cap/body separation: splitting using vacuum/air timing plus mechanical guidance.
● Dosing disc + tamping pins: forms a packed, repeatable metering volume before transfer.
● Dosator: uses a dosing tube/nozzle to pick up and transfer a powder charge.
● Locking integrity: consistency of cap–body engagement after closing.
● IPC: in-process checks (weight, closure, rejects) during the run.
A fully automatic capsule filler runs two flows in parallel: the capsule flow and the powder (fill) flow. When results drift, the root cause is typically separation timing, metering stability, or closing alignment—often triggered by capsule condition or powder behavior.

Feed/rectify → separate → close → discharge/reject
● Rectification affects how consistently shells enter separation and dosing.
● Separation relies on mechanical support plus vacuum/air timing; partial splits and shell stress often reappear later as closing defects.
● Closing depends on alignment and cleanliness. Powder on the cap/body interface is a common cause of loose lock and leakage.
● Reject/discharge should show a stable pattern over time; a rising reject rate usually signals buildup or drift upstream.
Condition → meter → transfer
Powder can bridge, aerate, segregate, pick up static, and shift bulk density with humidity. The dosing station translates that behavior into a repeatable dose through one of two common architectures:
● Dosing disc + tamping pins: compact powder into repeatable plugs (“slugs”) inside dosing bores, then transfer a metered volume.
● Dosator: capture and transfer a powder charge via a dosing tube/nozzle.
1. Separation timing: vacuum level, air timing, mechanical alignment.
2. Metering stability: powder bed consistency, tamp depth or dosator settings, speed window.
3. Closing alignment/force: guides, cleanliness, shell condition, closing geometry.
Layouts vary by manufacturer, but the functional sequence is consistent. At each station, focus on three questions: what is the station trying to do, what variables control it, and what failure looks like.
Controls: hopper flow, guide geometry, capsule size consistency, cleanliness.
Typical symptoms: misfeeds, scuffing, intermittent jams.
Fast checks: steady flow (no bursts); inspect guides for wear and powder buildup.
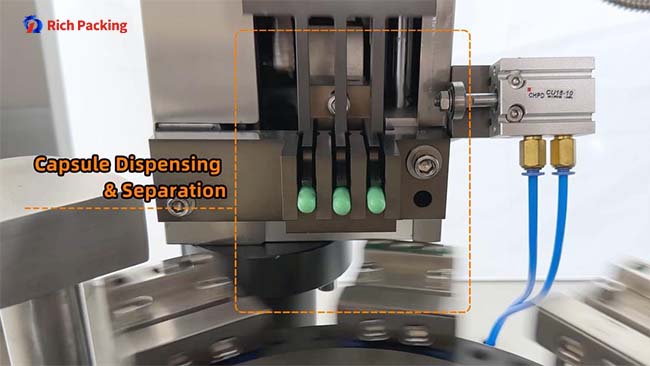
Controls: vacuum level, air timing, separation depth, turret speed, shell condition.
Typical symptoms: partial separation, cracked shells, caps not fully lifted.
Fast checks: validate clean splits at low speed, then ramp while watching shell damage and rejects.
Controls: mechanical alignment, holding stability, residue control.
Typical symptoms: body lift/wobble, inconsistent entry, powder “puffing.”
Fast checks: confirm bodies seat consistently and do not shift under vibration.
Controls: powder bed height, tamp depth, scraper/transfer cleanliness, turret speed.
Typical symptoms: weight drift, underfill, bridging, gradual buildup.
Fast checks: stabilize powder bed first, then adjust tamp depth, then validate at target speed.
Controls: fill depth, timing, powder conditioning, nozzle cleanliness.
Typical symptoms: charge inconsistency, smearing/buildup.
Fast checks: short-interval weights; inspect transfer surfaces for early residue.
Controls: guide alignment, closing force, station cleanliness, shell conditioning.
Typical symptoms: won’t close, loose lock, deformation, post-close leakage.
Fast checks: closure inspection on interval; if defects rise, re-check separation and interface contamination.

Controls: reject thresholds, sensor stability, discharge handling.
Typical symptoms: false rejects, missed defects, scuffing at discharge, rejects that climb over time.
Fast checks: trend reject patterns; confirm discharge handling does not damage good capsules.
|
Station |
Primary function |
Key variables |
Typical symptoms |
Fast verification |
|
Feeding/Rectification |
Orient capsules into turret |
Flow stability, guide wear, cleanliness |
Misfeeds, scuffing, jams |
Observe steady flow; inspect guides |
|
Separation |
Split cap and body |
Vacuum/air timing, speed, alignment |
Partial split, cracks |
Visual split check at low speed |
|
Body positioning |
Stabilize body for dosing |
Seating, alignment, residue |
Lift/wobble, inconsistent entry |
Watch seating consistency |
|
Dosing (disc/tamping) |
Meter packed volume |
Bed height, tamp depth, scrapers |
Weight drift, underfill |
Trend weights; change one variable |
|
Dosing (dosator) |
Meter charge via nozzle |
Depth, timing, conditioning |
Charge inconsistency |
Short-interval weights + cleanliness |
|
Closing |
Lock capsule |
Guides, force, contamination |
Won’t close, loose lock |
Closure checks on interval |
|
Discharge/Reject |
Remove defects, transfer product |
Thresholds, handling |
False rejects, scuffing |
Reject trend + discharge inspection |
Fill-weight control is where the rotary capsule filling machine working principle becomes measurable: stable powder conditioning, repeatable metering, and clean transfer. Most systems meter a repeatable volume/charge; final weight shifts when bulk density shifts or transfer efficiency shifts.
● Powder bed instability: inconsistent replenishment, bridging.
● Bulk density drift: aeration/vibration/humidity changes how powder packs.
● Segregation: blends separate, increasing variation (and content uniformity risk).
● Static and adhesion: powder sticks to dosing/transfer surfaces, causing slow drift.
For disc/tamping designs, repeatability usually improves fastest in this sequence:
1. Powder bed height/consistency
2. Tamping depth (small steps, one variable at a time)
3. Transfer cleanliness (scrapers/contact surfaces)
4. Speed window validation (ramp after weights are stable)
A dosator can perform well for certain powders and operating windows, but it still depends on powder condition and clean transfer. If drift appears, confirm powder behavior and residue first, then adjust dosator parameters.
● Weight trend (drift vs stable band)
● Start-up vs steady-state (first 10–20 minutes)
● Locking integrity checks
● Reject stability over time
Define sampling intervals, record results, and keep adjustments traceable. A simple cadence—more frequent at start-up, then steady—prevents most “surprises” later in the batch.
Higher speed reduces dwell time, so small instabilities show up faster as rejects and weight variation. In practice, qualification should focus on a stable speed window—the fastest speed that still holds weight and closure consistency over time.
● Separation consistency decreases (partial splits and shell stress increase).
● Powder bed replenishment becomes less stable (variation increases).
● Closing becomes less forgiving of alignment and contamination.
If rejects jump when you raise speed, slow back down and confirm which control domain breaks first (separation, metering, or closing).
Most dust/leakage originates from dosing transfer losses and powder contamination at the cap/body interface. Controls that commonly help:
● keep dosing/transfer surfaces clean (avoid gradual buildup)
● verify closing station cleanliness and guide alignment
● avoid overfill that prevents a clean lock
● add capsule polishing/dedusting when needed for downstream packaging or appearance

Hard capsule shells are sensitive to environment:
● too dry → brittle shells, cracking risk
● too humid → soft shells, deformation/loose lock risk
Stable conditioning near the line often improves locking integrity more than aggressive mechanical changes.
This section is a practical layer for first-time lines: minimum discipline plus fast fault isolation.
1. line clearance
2. batch record: key settings + IPC results + adjustments
3. cleaning procedure + changeover checklist
4. cross-contamination controls (dust management)
5. calibration records for IPC tools (balances, gauges if referenced)
6. safety basics (guards/interlocks/E-stops)
7. deviation handling triggers and documentation
8. training records for operators/maintenance
● stable operation in an agreed speed window
● IPC plan + weight trend evidence
● closure checks and defect handling approach
● reject consistency over time
● realistic cleaning access/time
● wear parts/spares list + lead times
|
Symptom |
Likely station/module |
Typical root cause |
Fix now |
Prevent next batch |
|
Weight variation increases |
Dosing / powder condition |
bed instability, density drift, residue |
stabilize bed; clean transfer; adjust tamp depth |
humidity control; conditioning routine; tighter IPC at start |
|
Underfilled/empty |
Separation or transfer |
partial separation, body not seated, bridging |
verify split; correct timing; clear bridging |
validate at low speed; capsule quality checks |
|
Won’t close / loose lock |
Closing + upstream |
misalignment, contamination, shell condition |
clean; verify guides; adjust closing force |
shell conditioning; cleaning cadence; verify rectification |
|
Leakage after closing |
Closing integrity |
loose lock, overfill, contamination |
confirm lock; reduce overfill; clean |
improve transfer cleanliness; polishing/dedusting if needed |
|
Cracks/deformation |
Separation/closing |
timing too aggressive; brittle/soft shells |
adjust timing; inspect guides |
environmental control; incoming QC |
|
Jams / bursts |
Feeding/rectification |
misfeeds, worn guides, buildup |
clear/clean; replace wear parts |
PM schedule; consistent capsule supply |
|
Rejects climb over time |
Dosing/closing buildup |
gradual residue, powder drift |
pause/clean; re-check IPC |
defined cleaning interval; trend rejects |
A fully automatic capsule filler is a station-timed cycle. When separation timing, metering stability, and closing alignment are controlled, fill weight and locking integrity become predictable and rejects stay stable. Used this way, the capsule filling machine working principle becomes a practical tool for evaluating equipment, qualifying a speed window, and isolating faults quickly.
1. How does a fully automatic capsule filling machine work?
It repeats rectification → separation → metering/transfer → closing/locking → discharge/reject.
2. Dosing disc/tamping pins vs dosator—what’s the difference?
Disc/tamping meters a packed volume; dosator meters a charge via nozzle. The best choice depends on powder behavior and the stability window you need.
3. Why does weight drift after start-up?
Common causes are bulk density drift and residue buildup on dosing/transfer surfaces.
4. Why do capsules fail to close?
Most often alignment, contamination at interfaces, or shell condition, sometimes traced back to weak separation.
5. Can one machine run powder and pellets?
Often yes with the correct dosing configuration and validation of transfer/closure/reject performance.
6. What should I prioritize in a FAT?
Evidence of control: weight trend, closure checks, reject stability, and realistic cleaning/changeover demonstration.
7. What is a practical start-up IPC cadence?
Sample more frequently during the first 10–20 minutes, then move to a steady interval once weight and rejects stabilize.
FDA – Process Validation: General Principles and Practices
https://www.fda.gov/regulatory-information/search-fda-guidance-documents/process-validation-general-principles-and-practices
USP – Dissolution Education Resources
https://www.usp.org/education/dissolution